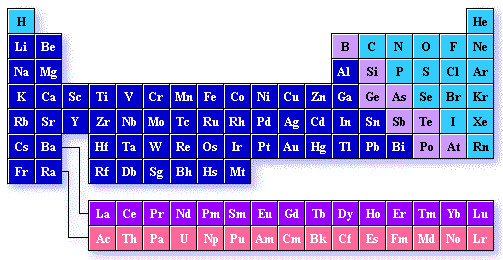
The periodic table is the most important chemistry reference there is. It arranges all the known elements in an informative array. Elements are arranged left to right and top to bottom in order of increasing atomic number.. This order generally coincides with increasing atomic mass The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies (in the unexcited state). The number of elements in a period increases as one traverses down the periodic table because as the energy level of the atom increases, the number of energy sub-levels per energy level increases.